Under the hood: The chemistry of cars - Cynthia Chubbuck
339,000 Views
6,308 Questions Answered
Let’s Begin…
There are over one billion cars in the world right now, getting people from point A to point B. But cars aren’t just a mode of transportation; they also teach an excellent lesson in chemistry. Cynthia Chubbuck navigates the intricate chemistry performed in our car engines that keep them from getting too hot or too cold.
Create and share a new lesson based on this one.
Additional Resources for you to Explore
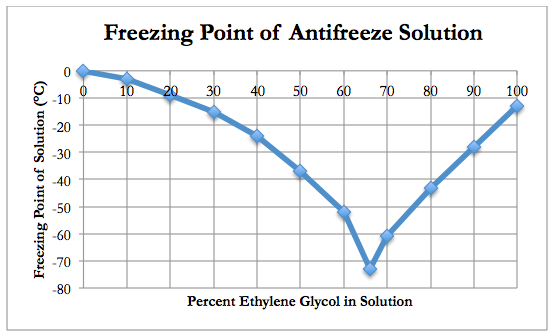
Based on the graph above, maximum protection from freezing the liquid in the cooling system occurs with a mixture of 66% ethylene glycol and 34% water. Based on the graph above, the minimum percentage of ethylene glycol that must be used in a radiator to prevent the fluid from freezing at –25°C is 40%.
There are two substances that are commonly used as the solute in a car radiator. Ethylene glycol (EG) is the traditional choice, but propylene glycol (PG) is gaining a foothold in the market. In comparing solutions using the two substances, an EG solution is more effective at lowering the freezing point and is better at transferring the heat away from the engine. A 50:50 mixture of EG and water freezes at -37°C while a similar solution of PG freezes at -34°C. PG has a greater viscosity than EG and in really cold climates a PG solution may not flow well through the engine compartment. Comparing the cost, PG is more expensive than EG. A one-gallon jug of 50% PG costs about two buck more than the same size container of EG.
One major concern with EG is that it is highly toxic to humans and pets. Because EG tastes sweet, a curious pet or toddler may be tempted to ingest the small spill that hit the driveway when the radiator was topped off. Some states now require that a bittering agent be added to EG products to discourage accidental consumption. On the other hand, PG has a much lower level of toxicity. The Food and Drug Association (FDA) has classified PG as “Generally Recognized as Safe” and has approved its use in food and food processing applications.
For a table comparing more of the properties of the two solutions, click here. At the bottom of the table there are links to additional information about EG and PG.
One of the problems with these traditional coolants is cavitation, the formation of vapor bubbles on the metal surface. While the engine is running there are hot spots where vapor bubbles form on the metal surface. These bubbles decrease coolant to metal contact, which decreases heat transfer. Another class of coolant addresses this problem by adding a surfactant. These substances interfere with the strong attractions between water molecules and lower the surface tension of the liquid. By lowering the surface tension the vapor bubbles shrink, reversing the effect. With smaller vapor bubbles there is more coolant to metal contact and better heat transfer. The main advantage of this approach is that it takes a small amount to do the job and with less solute, the excellent heat transfer properties of water are maintained. These products can be used alone or in conjunction with either EG or PG.
When a hot fluid runs through the engine, the metal components are vulnerable to corrosion. For this reason, all commercial antifreeze products contain corrosion inhibitors. The traditional formulations use Inorganic Additive Technology (IAT), which includes silicates, phosphates and/or borates. These ions quickly create a protective film over the metal surfaces. They also keep the solution alkaline, which is essential because acidic solutions speed up the corrosion process. With IAT additives, the cooling system needs to be flushed every three years or 36,000 miles. It’s not that the EG wears out, rather the corrosion inhibitors do.
Extended life formulas use Organic Acid Technology (OAT). OAT based corrosion inhibitors use a combination of carboxylic acid and fatty acids. They are more expensive but need only be changed every five years or 150,000 miles. These products should only be used in newer cars that do not have lead-based solder because they react with the lead weakening the soldered joints. Hybrid formulations called HOATs contain both organic and inorganic (silicates) corrosion inhibitors, which provide excellent corrosion resistance, but degrade more quickly.
To learn more about the different types of coolant, click here.
Technology now exists to recycle used antifreeze. As mentioned before, EG and PG do not wear out, the corrosion inhibitors do. The recycling process requires two steps. The goal of the first step is to remove any contaminants, especially any heavy metals that the fluid picked up as it flowed through the system, over and over again. After the ethylene glycol is purified, fresh additives are mixed in to restore the product to the original specifications. This process is cost effective, meaning it is cheaper to produce and use the recycled product than it is to buy new EG, and it saves petroleum-based resources.
To learn more about recycling antifreeze, click here.
Additional links:
1) http://www.engineeringtoolbox.com/ethylene-propylene-glycol-d_904.html
2) http://blog.chiltondiy.com/2013/06/which-antifreeze-is-right-for-your-vehicle/
3) http://www.epa.gov/epawaste/conserve/materials/antifree.htm
About TED-Ed Animations
TED-Ed Animations feature the words and ideas of educators brought to life by professional animators. Are you an educator or animator interested in creating a TED-Ed Animation? Nominate yourself here »
Meet The Creators
- Educator Cynthia Chubbuck
- Director Jay Hasrajani
- Editor Nathaniel Atcheson
- Producer Tyler Sadler, Matthew Chadwick, Hend Baghdady, Steve Shin
- Script Editor Alex Gendler
- Narrator Michelle Snow





